Vtama Cream
Generic name: tapinarof
Dosage form: cream
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
On This Page
Indications and Usage for Vtama Cream
VTAMA ® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults.
Vtama Cream Dosage and Administration
Apply a thin layer of Vtama Cream to affected areas once daily.
Wash hands after application, unless Vtama Cream is for treatment of the hands.
Vtama Cream is not for oral, ophthalmic, or intravaginal use.
Dosage Forms and Strengths
Cream, 1%
Each gram of Vtama Cream contains 10 mg of tapinarof in a white to off-white cream.
Contraindications
None.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two randomized, double-blind, multicenter, vehicle-controlled clinical trials (PSOARING 1 and PSOARING 2), 1025 adults with plaque psoriasis were treated with Vtama Cream or vehicle cream once daily for up to 12 weeks.
Subjects ranged in age from 18 to 75 years, with an overall median age of 51 years. The majority of subjects were white (85%) and male (57%); and 85% were non-Hispanic or Latino.
Table 1 presents adverse reactions that occurred in at least 1% of subjects treated with Vtama Cream, and for which the rate exceeded the rate for vehicle.
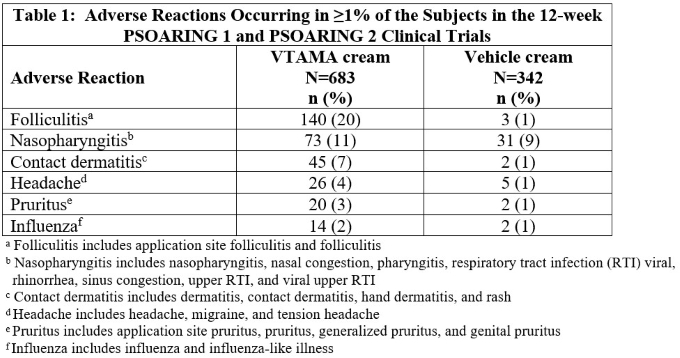
Two (0.3%) subjects using Vtama Cream developed urticaria. Adverse reactions leading to treatment discontinuation in >1% of subjects who received Vtama Cream were contact dermatitis (2.9%) and folliculitis (2.8%).
In an open label safety trial (PSOARING 3), 763 subjects were treated for up to an ad..



