Vyfemla
Generic name:norethindrone and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Nov 1, 2020.
On This Page
28 - DAY REGIMEN
Patients should be counseled that this product does not protect against HIV-infection (AIDS) and other sexually transmitted diseases.
Vyfemla Description
Vyfemla™ (norethindrone and ethinyl estradiol tablets USP) provides a continuous regimen for oral contraception derived from 21 light peach tablets composed of norethindrone and ethinyl estradiol to be followed by 7 white tablets of inert ingredients. The structural formulas are:
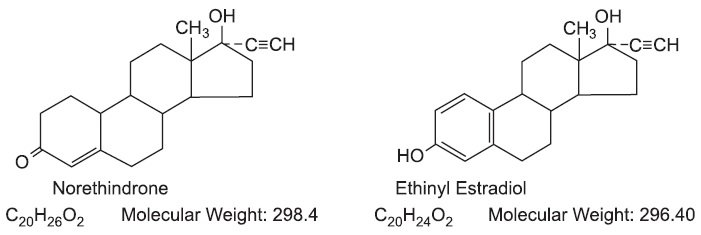
The light peach active Vyfemla (norethindrone and ethinyl estradiol tablets USP) contains 0.4 mg norethindrone and 0.035 mg ethinyl estradiol and contain the following inactive ingredients: FD&C yellow No. 6 (aluminum lake), lactose anhydrous, lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate. The white tablets in the 28 Day regimen contains only inert ingredients as follows croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
Vyfemla - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Indications and Usage for Vyfemla
Oral contraceptives are indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception.
Oral contraceptives are highly effective. Table 1 lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates.
...


