Xylon
Generic name:hydrocodone bitartrate and ibuprofen
Dosage form: tablet
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
The Xylon 10 brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Xylon Description
Each Xylon™ (hydrocodone bitartrate and ibuprofen tablets) contains either: Hydrocodone Bitartrate, USP 2.5 mg and Ibuprofen, USP 200 mg, Hydrocodone Bitartrate, USP 5 mg and Ibuprofen, USP 200 mg or Hydrocodone Bitartrate, USP 10 mg and Ibuprofen, USP 200 mg.
Xylon™ is supplied in a fixed combination tablet form for oral administration. Xylon™ combines the opioid analgesic agent, hydrocodone bitartrate, with the nonsteroidal anti-inflammatory (NSAID) agent, ibuprofen.
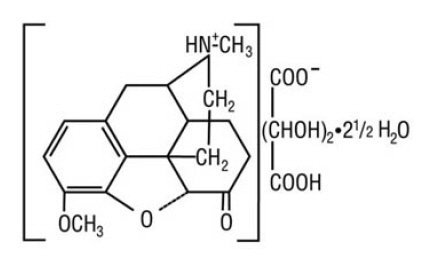
Hydrocodone bitartrate is a semisynthetic and centrally acting opioid analgesic. Its chemical name is: 4,5 α-epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1) hydrate (2:5). Its chemical formula is: C18H21NO3∙C4H6O6∙2½H2O, and the molecular weight is 494.50. Its structural formula is:
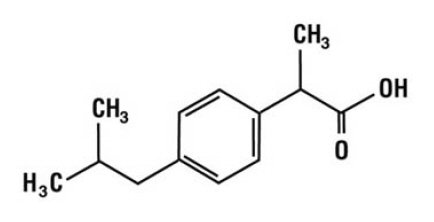
Ibuprofen is a nonsteroidal anti-inflammatory agent [non-selective COX inhibitor] with analgesic and antipyretic properties. Its chemical name is: (±)-2-(p-isobutylphenyl) propionic acid. Its chemical formula is: C13H18O2, and the molecular weight is: 206.29. Its structural formula is:
Inactive ingredients in Xylon™ 2.5 mg/200 mg and 5 mg/200 mg tablets include: carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polydextrose, pregelatinized starch and titanium diox...



