Bactroban Nasal Ointment
Generic name:mupirocin calcium
Dosage form: intranasal ointment
Drug class:Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
The Bactroban Nasal brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Bactroban Nasal Ointment Description
BACTROBAN Nasal (mupirocin calcium ointment, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (αE,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid, calcium salt (2:1), dihydrate.
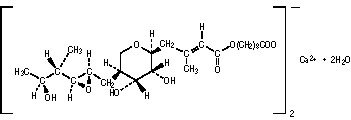
The molecular formula of mupirocin calcium is (C26H43O9)2Ca•2H2O, and the molecular weight is 1075.3. The molecular weight of mupirocin free acid is 500.6. The structural formula of mupirocin calcium is:
BACTROBAN Nasal is a white to off-white ointment that contains 2.15% w/w mupirocin calcium (equivalent to 2.0% pure mupirocin free acid) in a soft white ointment base. The inactive ingredients are paraffin and a mixture of glycerin esters (SOFTISAN® 649).
Bactroban Nasal Ointment - Clinical Pharmacology
Pharmacokinetics
Following single or repeated intranasal applications of 0.2 gram of BACTROBAN Nasal 3 times daily for 3 days to 5 healthy adult male subjects, no evidence of...