Zarontin Capsules
Generic name:ethosuximide
Dosage form: capsule
Drug class:Succinimide anticonvulsants
Medically reviewed by Drugs.com. Last updated on Nov 1, 2021.
On This Page
Zarontin Capsules Description
Zarontin (ethosuximide) is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alpha-methyl-succinimide, with the following structural formula:
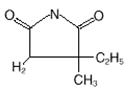
Each Zarontin capsule contains 250 mg ethosuximide, USP. Also contains: polyethylene glycol 400, NF. The capsule contains D&C yellow No. 10; FD&C red No. 3; gelatin, NF; glycerin, USP; and sorbitol.
Zarontin Capsules - Clinical Pharmacology
Ethosuximide suppresses the paroxysmal three cycle per second spike and wave activity associated with lapses of consciousness which is common in absence (petit mal) seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli.
Indications and Usage for Zarontin Capsules
Zarontin is indicated for the control of absence (petit mal) epilepsy.
CONTRAINDICATION
Ethosuximide should not be used in patients with a history of hypersensitivity to succinimides.
Warnings
Blood Dyscrasias
Blood dyscrasias, including some with fatal outcome, have been reported to be associated with the use of ethosuximide; therefore, periodic blood counts should be performed. Should signs and/or symptoms of infection (e.g., sore throat, fever) develop, blood counts should be considered at that point.
Drug-Induced Immune Thrombocytopenia
Drug-induced immune thrombocytopenia (DITP) has been reported with ethosuximide. In the reported cases, the onset of symptoms occurred 1 to 3 weeks after initiation of ethosuximide; one patient had recurrence of symptoms within 1 day of a subsequent re-challenge with the drug. In those cases in which the platelet count was specified, the nadir was 2,000 and 3,000/mm3. When DITP is suspected, discontinue Zarontin, monitor ser...



