Zazole Vaginal Cream
Generic name:terconazole
Dosage form: vaginal cream
Drug class:Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
Rx only
The Zazole brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Zazole Vaginal Cream Description
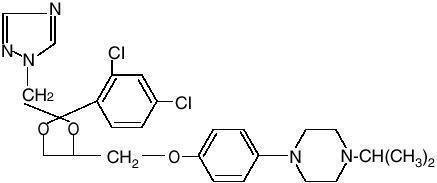
Zazole® Vaginal Cream 0.4% (terconazole vaginal cream 0.4%) is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1-H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. The structural formula of terconazole is as follows:
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
Zazole Vaginal Cream - Clinical Pharmacology
Following intravaginal administration of terconazole in humans, absorption ranged from 5-8 % in three hysterectomized subjects and 12-16 % in two non-hysterectomized subjects with tubal ligations.
Following oral (30 mg) administration of 14C-labelled terconazole, the harmonic half-life of elimination from the blood for the parent terconazole was 6.9 hours (range 4.0-11.3). Terconazole is extensively metabolized; the plasma AUC for terconazole compared to the AUC for total radioactivity was 0.6 %. Total radioactivity was eliminated from the blood with a harmonic half-life of 52.2 hours (range 44-60). Excretion of radioactivity was both by renal (32-56 %) and fecal (47-52 %) routes.
In vitro, terconazole is highly protein bound (94.9 %) and the degree of binding is independent of dru...