Zazole Vaginal Cream 0.8%
Generic name:terconazole
Dosage form: vaginal cream
Drug class:Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
Rx Only
The Zazole brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Zazole Vaginal Cream 0.8% Description
Zazole® Vaginal Cream 0.8% (terconazole vaginal cream 0.8%) is a white to off-white, water washable cream for intravaginal administration containing 0.8% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1-H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. The structural formula of terconazole is as follows: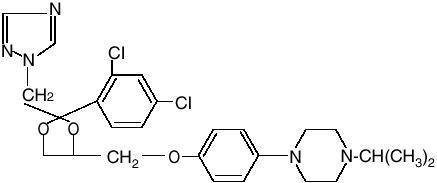
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
Zazole Vaginal Cream 0.8% - Clinical Pharmacology
Following intravaginal administration of terconazole in humans, absorption ranged from 5-8% in three hysterectomized subjects and 12-16% in two non-hysterectomized subjects with tubal ligations.
Following daily intravaginal administration of 0.8% terconazole 40 mg (0.8% cream x 5 g) for seven days to normal humans, plasma concentrations were low and gradually rose to a daily peak (mean of 5.9 ng/mL or 0.006 mcg/mL) at 6.6 hours. Results from similar studies in patients with vulvovaginal candidiasis indicate that the slow rate of absorption, the lack of accumulation, and the mean peak plasma concentration of terconazole was not different from that observed in healthy women. The absorption characteristics of terconazole 0.8% in pregnant or non-pregnant patients with vulvovaginal candidiasis were also similar to those found in normal volunteers.
Following oral (30 mg) ...



