Aclaro Emulsion
Generic name:hydroquinone
Dosage form: topical emulsion
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
The Aclaro brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Description
Rx only
For topical use only
Not for ophthalmic use
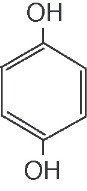
Hydroquinone is 1,4-benzenediol. Hydroquinone is structurally related to monobenzone. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol with a pKa of 9.96. Chemically, h ydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6 H6 O2; molecular weight 110.1.The structural formula is:
Active Ingredients: hydroquinone USP 4% Other Ingredients: ascorbic acid, benzyl alcohol, butyl methoxydibenzoylmethane, C12-15 alkyl benzoate, cetearyl octanoate, cetyl alcohol, cetyl esters, cetyl palmitate, DEA cetyl phosphate, dimethicone, disodium EDTA, ethylhexyl methoxycinnamate, glycerin, glycolic acid, ammonium glycolate, hydroxyethylcellulose, phenoxyethanol, purified water, sodium metabisulfite, and stearic
acid.
Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3- (3,4-dihydroxyphenyl) alanine (dopa) and suppression of other melanocyte metabolic processes. 2
Indications and Usage
Aclaro® is indicated for the gradual treatment of ultraviolet induced dyschromia and discoloration resulting rom the use of oral contraceptives, pregnancy, hormone replacment therapy, or skin trauma.



