Zolpak
Generic name: econazole nitrate
Dosage form: cream
Drug class:Topical antifungals
Medically reviewed by Drugs.com. Last updated on Nov 1, 2020.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Rx Only
For Topical Use Only
Zolpak Description
Econazole Nitrate Cream contains the antifungal agent, econazole nitrate 1% in a water miscible base consisting of pegoxol 7 stearate, peglicol 5 oleate, mineral oil, benzoic acid, butylated hydroxyanisole, and purified water. The white to off-white soft cream is for topical use only.
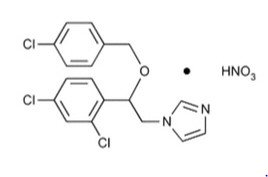
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl) methoxy}-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole mononitrate. Its structure is as follows:
Zolpak - Clinical Pharmacology
After topical application to the skin of normal subjects, systemic absorption of econazole nitrate is extremely low. Although most of the applied drug remains on the skin surface, drug concentrations were found in the stratum corneum which, by far, exceeded the minimum inhibitory concentration for dermatophytes. Inhibitory concentrations were achieved in the epidermis and as deep as the middle region of the dermis. Less than 1% of the applied dose was recovered in the urine and feces.
Microbiology
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
| Dermatophytes | Yeasts |
| Epidermophyton floccosum | Candida albicans |
| Microsporum audouini | Malassezia furfur |
| Microsporum canis | |
| Microsporum gypseum | |
| Trichophyton mentagrophytes | |
| Trichophyton rubrum | |
| Trichophyton tonsurans |



