Zypram Cream
Generic name:hydrocortisone acetate and pramoxine hydrochloride
Dosage form: cream
Drug class:Anorectal preparations
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
The Zypram brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Zypram Cream Description
ZyPram™ Cream is a topical preparation containing hydrocortisone acetate 2.35% in a cream base and pramoxine hydrochloride 1% in a hydrophilic cream base1. Hydrocortisone acetate has a chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11,17-dihydroxy-(11β)-. It has the following structural formula:
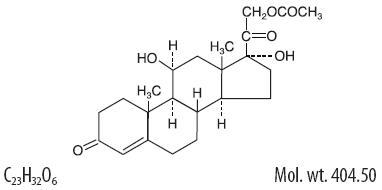
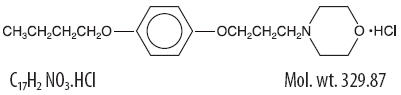
Pramoxine hydrochloride has chemical name 4-[3-(4-butoxyphenoxy) propyl]morpholine hydrochloride, and has the following structure:
Active ingredients: Hydrocortisone Acetate 2.35% and Pramoxine Hydrochloride 1%.
Inactive ingredients: Ammonium Acryloyldimethyltaurate/VP-Copolymer, Benzyl Alcohol, Cetearyl Alcohol, Cetearyl Olivate (&) Sorbitan Olivate, PEG-12 Glyceryl Distearate=GDS-12, PEG-12 Glyceryl Dimyristate=GDM-12, Glycerine, Glyceryl Stearate, Methyl Paraben, PEG 100 Stearate, Polysorbate 60, Propylene Glycol, Purified Water, Sodium Lauryl Sulfate, White Petrolatum.
Cleansing Wipe (2.2 grams solution per wipe) Contains:
Citric Acid . . . . . . . . . . . . . . . . . . . . . . . 22 mg
Aloe Vera . . . . . . . . . . . . . . . . . . . . . . . 11 mg
Vitamin E (dl-tocopheryl acetate) . . . . . . 2.2 mg
...



