Benzoyl Peroxide Foam
Dosage form: aerosol, foam
Drug class:Topical acne agents
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Benzoyl Peroxide Foam Description
Each gram of Benzoyl Peroxide Short Contact Foam contains 9.8% benzoyl peroxide in an aqueous based emollient foam vehicle containing BHT, C12-15 alkyl benzoate, cetearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10. Also contains: Propellant HFA-134a (1,1,1,2-tetrafluoroethane).
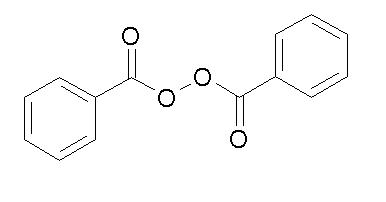
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
Benzoyl Peroxide Foam - Clinical Pharmacology
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide, is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
Indications and Usage for Benzoyl Peroxide Foam
Benzoyl Peroxide Short Contact Foam is indicated for use in the topical treatment of mild to moderate acne vulgaris.
Contraindications
Benzoyl Peroxide Short Contact Foam should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product. Discontinue use if hypersensitivity is observed.
Warnings
FOR EXTERNAL USE ONLY....