Buscopan
Generic name: n-butylscopolammonium bromide injection
Dosage form: FOR ANIMAL USE ONLY
On This Page
Approved by FDA under NADA # 141-228
Antispasmodic (spasmolytic) and anticholinergic drug for intravenous use in horses only.
Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
Buscopan Injectable Solution is an antispasmodic (spasmolytic) and anticholinergic drug which suppresses spasms of the digestive system.
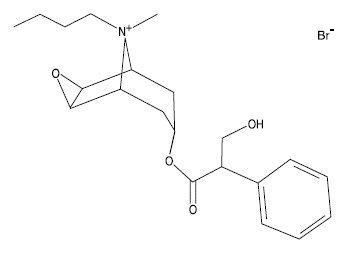
The chemical name for the active constituent of Buscopan is N-butylscopolammonium bromide. It is a water soluble, white crystalline substance with a molecular weight of 440.40. Each mL of Buscopan contains 20 mg N-butylscopolammonium bromide, 1.8 mg methylparaben, 0.2 mg propylparaben, 6.0 mg sodium chloride, and water for injection. The chemical structure is:
Indications:
Buscopan is indicated for the control of abdominal pain (colic) associated with spasmodic colic, flatulent colic, and simple impactions in horses.
Dosage and Administration:
Administer a single injection of 0.3 mg/kg body weight (0.14 mg/lb), slowly IV. This is equivalent to 30 mg N-butylscopolammonium bromide per 100 kg (220 pounds) bodyweight or 1.5 mL of Buscopan per 100 kg (220 pounds) bodyweight.
Body Weight (nearest 100 pounds) | Dose (mL) | Body Weight (nearest 100 pounds) | Dose (mL) |
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
|