Capozide
Generic name:captopril and hydrochlorothiazide
Dosage form: Tablets, USP
Drug class:ACE inhibitors with thiazides
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, Capozide® should be discontinued as soon as possible. See WARNINGS: Captopril: Fetal/Neonatal Morbidity and Mortality.
The Capozide brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Capozide Description
Capozide® (captopril and hydrochlorothiazide tablets, USP) for oral administration combines two antihypertensive agents: captopril and hydrochlorothiazide. Captopril, the first of a new class of antihypertensive agents, is a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II. Hydrochlorothiazide is a benzothiadiazide (thiazide) diuretic-antihypertensive. Capozide tablets are available in four combinations of captopril with hydrochlorothiazide: 25 mg with 15 mg, 25 mg with 25 mg, 50 mg with 15 mg, and 50 mg with 25 mg. Inactive ingredients: microcrystalline cellulose, colorant (FD&C Yellow No. 6), lactose, magnesium stearate, pregelatinized starch, and stearic acid.
Captopril is designated chemically as 1-[(2S)-3-mercapto-2-methylpropionyl]-L-proline; hydrochlorothiazide is 6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Graphic formulas:
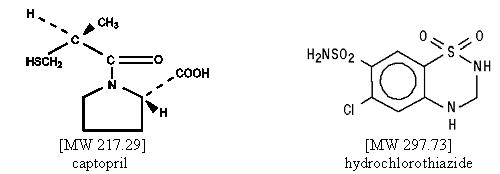
Captopril is a white to off-white crystalline powder that may have a slight sulfurous odor; it is soluble in water (approx. 160 mg/mL), methanol, and ethanol and sparingly soluble in chloroform and ethyl acetate.
Hydrochlorot...



