Dosage form: tablet
Drug class:Angiotensin Converting Enzyme Inhibitors
Medically reviewed by Drugs.com. Last updated on Jun 1, 2021.
On This Page
- •
- When pregnancy is detected, discontinue Captopril Tablets as soon as possible.
- •
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity
Captopril Tablets Description
Captopril Tablets, USP are a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II.
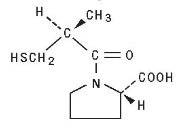
Captopril is designated chemically as 1-[(2S)-3-mercapto-2-methylpropionyl] -L-proline [MW 217.28] and has the following structure:
Captopril USP is a white to off-white crystalline powder that may have a slight sulfurous odor; it is soluble in water (approx. 126 mg/mL), methanol, and ethanol and sparingly soluble in chloroform and ethyl acetate.
Captopril Tablets, USP are available in potencies of 12.5 mg, 25 mg, 50 mg, and 100...