Carbastat
Generic name:carbachol
Dosage form: injection
Drug class:Ophthalmic glaucoma agents
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
Carbastat®
(CARBACHOL INTRAOCULAR SOLUTION, USP) 0.01%
On This Page
DESCRIPTION
Carbastat®(Carbachol Intraocular Solution, USP) 0.01% is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the structural formula:
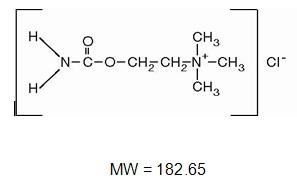
Established name: Carbachol
Chemical name: Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,N -trimethyl-, chloride.
Each mL contains: Active: Carbachol 0.01%.
Inactive: Sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dihydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid to adjust pH (5.0-7.5) and water for injection USP.
Carbastat - Clinical Pharmacology
Carbachol is a potent cholinergic (parasympathomimetic) agent which produces constriction of the iris and ciliary body resulting in reduction in intraocular pressure. The exact mechanism by which carbachol lowers intraocular pressure is not precisely known.
Indications and Usage for Carbastat
Intraocular use for obtaining miosis during surgery. In addition, Carbastat® (Carbachol Intraocular Solution USP) reduces the intensity of intraocular pressure elevation in the first 24 hours after cataract surgery.
Contraindications
Should not be used in those persons showing hypersensitivity to any of the components of this preparation.
Warnings
For single-dose intraocular use only. Discard unused portion. Intraocular carbachol 0.01% should be used with caution in patients with acute cardiac failure, bronchial asthma, peptic ulcer, hyperthyroidism, G.I. spasm, urinary tract obstruction and Parkinson's disease.
Precautions
Carcinogenesis
Studies in animals to evaluate the carcinogenic potential have not been conducted.
Pregnancy: Category C.
There are no adequate and well controlled studies in pregnant women. Carbas...



