Chlorothiazide Injection
Dosage form: injection, powder, lyophilized, for solution
Drug class:Thiazide diuretics
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
novaplusTM+
Rx Only
DESCRIPTION
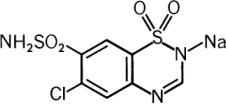
Chlorothiazide Sodium for Injection, USP is a diuretic and antihypertensive. It is 6-chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide monosodium salt and its molecular weight is 317.71. Its empirical formula is C7H5ClN3NaO4S2 and its structural formula is:
Chlorothiazide Sodium for Injection, USP is a sterile lyophilized white powder and is supplied in a vial containing: Chlorothiazide sodium equivalent to chlorothiazide 500 mg, and the inactive ingredient mannitol 250 mg with sodium hydroxide to adjust pH.
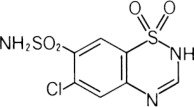
Chlorothiazide is a diuretic and antihypertensive. It is 6-chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H6ClN3O4S2 and its structural formula is:
It is a white, or practically white, crystalline powder with a molecular weight of 295.72, which is very slightly soluble in water, but readily soluble in dilute aqueous sodium hydroxide. It is soluble in urine to the extent of about 150 mg per 100 mL at pH 7.
CLINICAL PHARMACOLOGY
The mechanism of the antihypertensive effect of thiazides is unknown. Chlorothiazide does not usually affect normal blood pressure.
Chlorothiazide affects the distal renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage all thiazides are approximately equal in their diuretic efficacy.
Chlorothiazide increases excretion of sodium and chloride in approximately equivalent amounts. Natriuresis may be accompanied by some loss of potassium and bicarbonate.
After oral u..