Cidofovir Injection
Generic name: cidofovir dihydrate
Dosage form: injection, solution
Drug class:Purine nucleosides
Medically reviewed by Drugs.com. Last updated on Apr 1, 2021.
On This Page
RENAL IMPAIRMENT IS THE MAJOR TOXICITY OF Cidofovir Injection. CASES OF ACUTE RENAL FAILURE RESULTING IN DIALYSIS AND/OR CONTRIBUTING TO DEATH HAVE OCCURRED WITH AS FEW AS ONE OR TWO DOSES OF Cidofovir Injection. TO REDUCE POSSIBLE NEPHROTOXICITY, INTRAVENOUS PREHYDRATION WITH NORMAL SALINE AND ADMINISTRATION OF PROBENECID MUST BE USED WITH EACH CIDOFOVIR INFUSION. RENAL FUNCTION (SERUM CREATININE AND URINE PROTEIN) MUST BE MONITORED WITHIN 48 HOURS PRIOR TO EACH DOSE OF Cidofovir Injection AND THE DOSE OF Cidofovir Injection MODIFIED FOR CHANGES IN RENAL FUNCTION AS APPROPRIATE (SEE DOSAGE AND ADMINISTRATION). Cidofovir Injection IS CONTRAINDICATED IN PATIENTS WHO ARE RECEIVING OTHER NEPHROTOXIC AGENTS.
NEUTROPENIA HAS BEEN OBSERVED IN ASSOCIATION WITH Cidofovir Injection TREATMENT. THEREFORE, NEUTROPHIL COUNTS SHOULD BE MONITORED DURING Cidofovir Injection THERAPY.
Cidofovir Injection IS INDICATED ONLY FOR THE TREATMENT OF CMV RETINITIS IN PATIENTS WITH ACQUIRED IMMUNODEFICIENCY SYNDROME.
IN ANIMAL STUDIES CIDOFOVIR WAS CARCINOGENIC, TERATOGENIC AND CAUSED HYPOSPERMIA (SEE CARCINOGENESIS, MUTAGENESIS, & IMPAIRMENT OF FERTILITY).
Cidofovir Injection Description
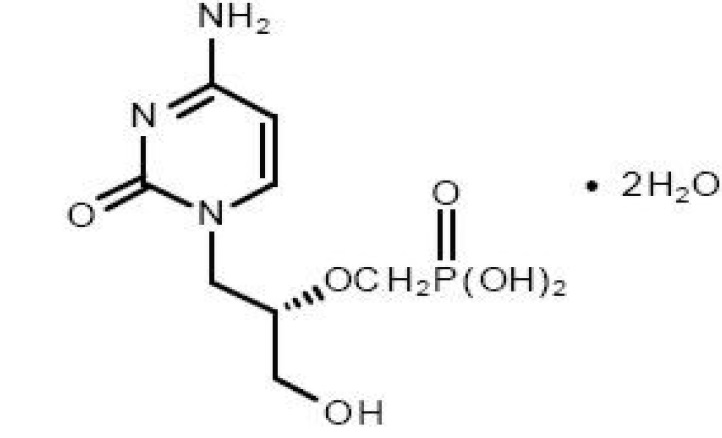
The chemical name of cidofovir USP is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P•2H2O and a molecular weight of 315.22 (279.19 for anhydrous). The chemical structure is:
Cidofovir USP is a white crystalline powder with an aqueous solubility of ≥ 170 mg...



