Covid-19 Vaccine Novavax
Generic name: nvx-cov2373
Dosage form: injection, suspension
Drug class:Viral vaccines
Medically reviewed by Drugs.com. Last updated on Mar 1, 2022.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
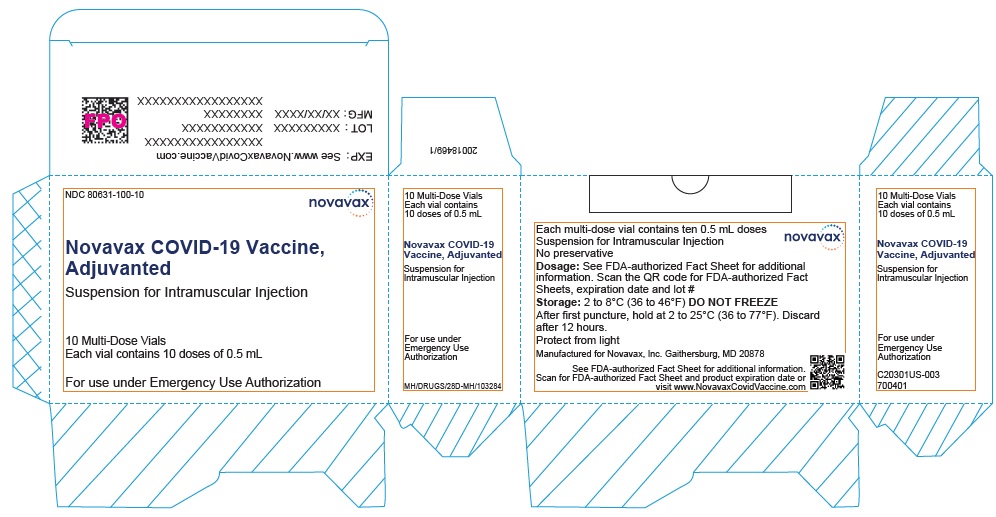
NDC 80631-100-10 novavax
10 Multi-Dose Vials
(10 doses of 0.5mL)
novavax
COVID-19 Vaccine, Adjuvanted
Suspension for
Intramuscular Injection
For use under Emergency Use Authorization
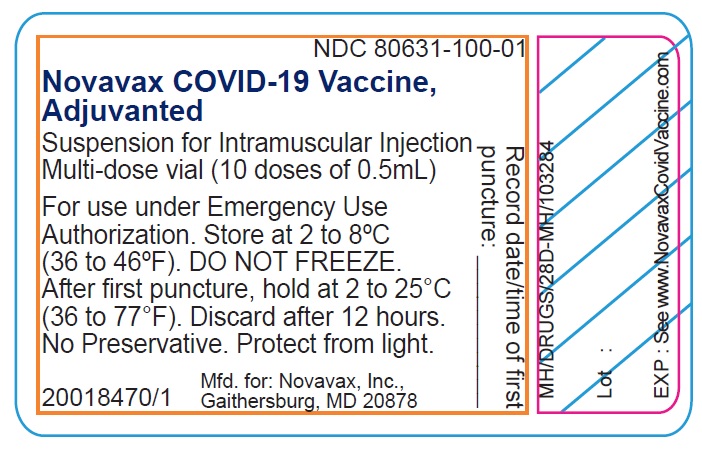
novavax NDC 80631-100-01
COVID-19 Vaccine, Adjuvanted
Suspension for Intramuscular Injection
Multi-dose vial (10 doses of 0.5mL)
Record date/time of first
puncture:
No Preservative
Date: Time:
For use under Emergency Use Authorization
See FDA-authorized Fact Sheet for dosage and
administration. Store at 2 to 8ºC (36 to 46ºF)
DO NOT FREEZE. Discard 6 hours after first
puncture when stored at 2 to 25°C (36 to 77°F)
Keep the vial in carton to protect from light
EXP: See www.NovavaxCovidVaccine.com
Manufactured for: Novavax, Inc., Gaithersburg, MD 20878
xxxxxx-xxx GTIN 0123456789
novavax
| NOVAVAX COVID-19 VACCINE, ADJUVANTED nvx-cov2373 injection, suspension | ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||



