Cyclopentolate
Generic name: Cyclopentolate hydrochloride
Dosage form: ophthalmic solution
Drug class:Mydriatics
Medically reviewed by Drugs.com. Last updated on May 1, 2021.
On This Page
DESCRIPTION
Cyclopentolate Hydrochloride Ophthalmic Solution USP is an anticholinergic prepared as a sterile, borate buffered, solution for topical ocular use. It is supplied in three strengths. The active ingredient is represented by the structural formula:
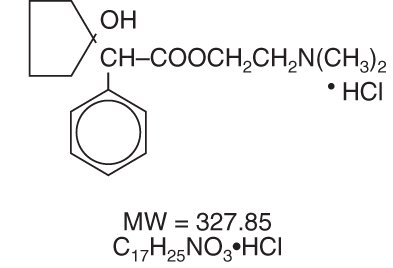
Established name: Cyclopentolate Hydrochloride
Chemical name: 2-(Dimethylamino)ethyl 1-hydroxy-a-phenylcyclopentaneacetate hydrochloride
Each mL of Cyclopentolate hydrochloride ophthalmic solution, USP contains: Active: Cyclopentolate hydrochloride 0.5%, 1% or 2%. Preservative: benzalkonium chloride 0.01%. Inactives: boric acid, edetate disodium, potassium chloride (except 2% strength), sodium carbonate and/or hydrochloric acid (to adjust pH), purified water. The pH range is between 3.0 and 5.5.
CLINICAL PHARMACOLOGY
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia). It acts rapidly, but has a shorter duration than atropine.
Maximal cycloplegia occurs within 25 to 75 minutes after instillation. Complete recovery of accommodation usually takes 6 to 24 hours. Complete recovery from mydriasis in some individuals may require several days. Heavily pigmented irides may require more doses than lightly pigmented irides.
INDICATIONS AND USAGE
Cyclopentolate hydrochloride is used to produce mydriasis and cycloplegia.
CONTRAINDICATIONS
Should not be used if the patient is hypersensitive to any component of this preparation.
WARNINGS
For topical ophthalmic use. Not for injection. This preparation may cause Central Nervous System (CNS) disturbances. This is especially true in younger age groups...



