Cyred EQ
Generic name:desogestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Apr 1, 2022.
On This Page
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including Cyred EQ®, should not be used by women who are over 35 years of age and smoke.
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Cyred EQ Description
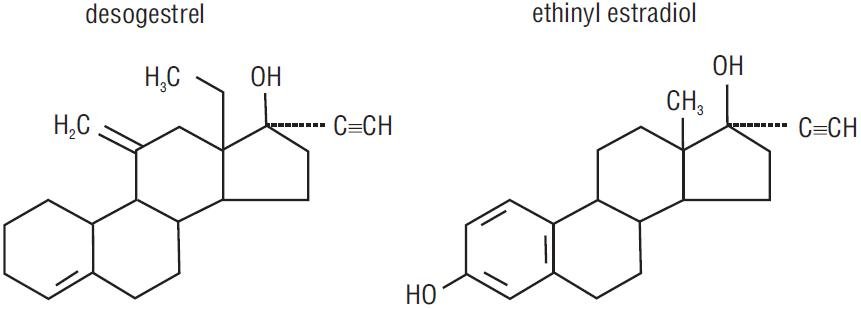
Cyred EQ® tablets provide an oral contraceptive regimen of 21 white to off-white round tablets each containing 0.15 mg desogestrel (13-ethyl-11-methylene-18,19-dinor-17 alpha-pregn-4-en-20-yn-17-ol) and 0.03 mg ethinyl estradiol USP (19-nor-17 alpha-pregna-1,3,5 (10)-trien-20-yne-3,17,diol). Inactive ingredients include colloidal silicon dioxide, lactose monohydrate, potato starch, povidone, stearic acid, and vitamin E. Each green tablet contains the following inactive ingredients: anhydrous lactose, croscarmellose sodium, FD&C Blue No.2 Aluminum Lake, ferric oxide yellow, magnesium stearate, microcrystalline cellulose, and povidone.
Cyred EQ meets USP Dissolution Test 2.
Cyred EQ - Clinical Pharmacology
Pharmacodynamics
Combined oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus, which increase the difficulty of sperm entry into the uterus, and changes in the endometrium which reduce the likelihood of...



