Daypro Alta
Generic name:oxaprozin potassium
Dosage form: Tablets
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
- Boxed Warning
- Description
- Clinical Pharmacology
- Clinical Studies
- Indications and Usage
- Contraindications
- Warnings
- Precautions
- Patient Counseling Information
- Drug Interactions
- Adverse Reactions/Side Effects
- Overdosage
- Dosage and Administration
- How Supplied/Storage and Handling
- Supplemental Patient Material
- Medication Guide
Cardiovascular Risk
- NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patient's with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS).
- Daypro Alta™ is contraindicated for treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
- NSAID's cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).
Daypro Alta Description
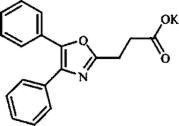
Daypro Alta (oxaprozin potassium tablets) is a member of the propionic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each blue, capsule-shaped tablet contains oxaprozin potassium (678mg equivalent to 600mg of oxaprozin) for oral administration. The chemical name for oxaprozin potassium is 4,5-diphenyl-2-oxazolepropionic acid, potassium salt. Its empirical formula is C18H14NO3K, and molecular weight is 331. Oxaprozin potassium is a white to off white powder with a melting point of 215°C. It is slightly soluble in alcohol and very soluble in water. The PK in water is 9.7.
It has the following structural formula:
Inactive ingredients in Daypro Alta ta...



