Delos Liquid
Generic name:benzoyl peroxide
Dosage form: topical liquid
Drug class:Topical acne agents
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Delos Liquid Description
Delos™ (3.5% benzoyl peroxide) cleanser is a topical preparation containing benzoyl peroxide, for use in the treatment of acne vulgaris. Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic agent. Benzoyl peroxide (C14H10O4) is represented by the following chemical structure:
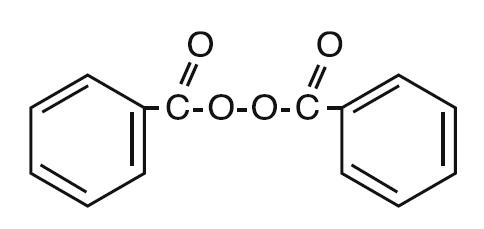
Delos™ (3.5% benzoyl peroxide) cleanser contains benzoyl peroxide USP 3.5% as the active ingredient in a formulation consisting of: Cetearyl Alcohol, Ceteareth-20, Citric Acid, Cocamidopropyl Betaine, Cocamine Oxide, Disodium Laureth Sulfosuccinate, Edetate Disodium, Magnesium Aluminum Silicate, Methylparaben, PEG-15 Pentaerythrityl Tetrastearate, PEG-6 Caprylic/Capric Glycerides, PEG-150 Stearate, 2-Phenoxyethanol, Poloxamer 182, Polyethylene, Sodium Polyacrylate, PPG-20 Methyl Glucose Ether, Propylene Glycol, Purified Water, and Xanthan Gum.
Delos Liquid - Clinical Pharmacology
The mechanism of action of benzoyl peroxide is not totally understood but its antibacterial activity against Propionibacterium acnes is thought to be a major mode of action, in addition, patients treated with benzoyl peroxide show a reduction in lipids and free fatty acids, and mild desquamation (drying and peeling activity) with simultaneous reduction in comedones and acne lesions. Little is known about the percutaneous penetration, metabolism, and excretion of benzoyl peroxide, although it has been shown that benzoyl peroxide absorbed by the skin is metabolized to benzoic acid and then excreted as benzoate in the urine. There is no evidence of systemic toxicity caused by benzoyl peroxide in humans.



