Delyla
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
Delyla Description
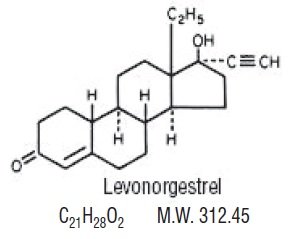
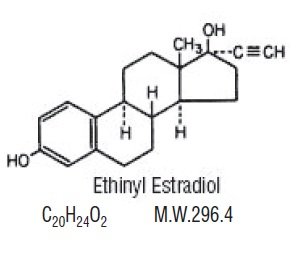
Each active, white tablet (21) contains 0.1 mg of levonorgestrel, USP, d(-)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one, a totally synthetic progestogen, and 0.02 mg of ethinyl estradiol, USP, 17α-ethinyl-1,3,5(10)-estratriene-3, 17β-diol. The inactive ingredients present are lactose monohydrate, magnesium stearate, microcrystalline cellulose, polacrilin potassium and Aqua Polish White 014.17 MS which contains hydroxypropylcellulose, hydrogenated cottonseed oil, hydroxypropylmethylcellulose, talc, and titanium dioxide.
Each inactive, yellow tablet (7) contains the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, polacrilin potassium and Aqua Polish Yellow 024.15 MS which contains hydroxypropylcellulose, hydrogenated cottonseed oil, hydroxypropylmethylcellulose, ferric oxide red, ferric oxide yellow, talc, and titanium dioxide.
Delyla - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).