Dendrid
Generic name: idoxuridine
Dosage form: ophthalmic solution
Drug class:Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
On This Page
DESCRIPTION
Dendrid® (idoxuridine) is an antiviral chemotherapeutic agent prepared in a sterile buffered isotonic solution. The active ingredient is represented by the chemical structure:
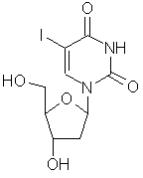
Established name:
Idoxuridine
Chemical name:
Uridine, 2’-deoxy-5-iodo-
Each ml contains: Active: Idoxuridine 0.1%. Preservative: Benzalkonium Chloride 0.01%. Inactive: Boric Acid, Edetate Disodium, Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH), Purified Water. DM-01
CLINICAL PHARMACOLOGY
Herpes simplex virus utilizes thymidine in the synthesis of deoxyribonucleic acid (DNA), a metabolite necessary for reproduction. Idoxuridine is identical in chemical structure to thymidine except that the 5-methyl group is replaced by iodine. When idoxuridine is substituted for thymidine in DNA, the cell is unable to utilize the DNA and reproduction ceases.
INDICATIONS AND USAGE
For the treatment of keratitis caused by the virus of herpes simplex.
CONTRAINDICATION
Hypersensitivity to the active ingredient or any other component of this drug.
PRECAUTIONS
For topical use only. Do not exceed the frequency or duration of recommended dosage. The incidence of some adverse reactions increases with prolonged use. Idoxuridine is not effective in corneal inflammations following herpes simplex keratitis in which the virus is not present. Some strains of herpes simplex appear resistant to the action of Idoxuridine.
USAGE IN PREGNANCY
Idoxuridine should be administered with caution in pregnancy or in women of childbearing potential. Idoxuridine has been reported to cross the placental barrier and to produce fetal malformations in rabbits when administered topically to the eyes of pregnant females in doses similar to those used clinically. Idoxuridine has also been reported to produce fetal malformations in the rat after intraperitoneal and oral administration and in the mouse after subcutaneous administration. Nursing should not be performed while a patient is undergoing IDU treatment as the drug and metabolites may be excreted in human milk.
MUTAGENIC POTENTIAL
Idoxuridine has been reported to cause chromosome aberrations in mi...



