Dermazene
Generic name:hydrocortisone and iodoquinol
Dosage form: cream
Drug class:Topical steroids with anti-infectives
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx only
Dermazene Description
Each gram of Dermazene® Cream 1% contains 10 mg of hydrocortisone and 10 mg of iodoquinol in a cream base of purified water, propylene glycol, cerasynt SE, amerchol L101, isopropyl palmitate, cetyl alcohol, arlacel 60, myrj 52, tween 60, sorbic acid, methyl paraben and propyl paraben.
Chemically, hydrocortisone is [Pregn-4-ene-3,20-dione, 11, 17, 21-trihydroxy-(11β)-] with the molecular formula (C21H30O5) and is represented by the following structural formula:
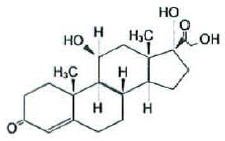
and iodoquinol, 5,7-diiodo-8-quinolinol (C9H5I2NO) is represented by the following structure:
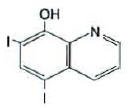
Hydrocortisone is an anti-inflammatory and antipruritic agent, while iodoquinol is an antifungal and antibacterial agent.
Dermazene - Clinical Pharmacology
Hydrocortisone has anti-inflammatory, antipruritic and vasoconstrictor properties. The mechanism of anti-inflammatory activity is unclear. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Iodoquinol has both antifungal and antibacterial properties.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Hydrocortisone can be absorbed from normal intact skin. Inflammation and/or other inflammatory disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids.
Once absorbed through the ski...



