Doxapram
Generic name: Doxapram hydrochloride
Dosage form: injection
Drug class:CNS stimulants
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
NOT FOR USE IN NEONATES
CONTAINS BENZYL ALCOHOL
Rx ONLY
Doxapram Description
Doxapram Hydrochloride Injection USP, is a clear, colorless, sterile, non-pyrogenic, aqueous solution with pH 3.5 to 5, for intravenous administration.
Each mL contains Doxapram hydrochloride 20 mg, benzyl alcohol (as preservative) 0.9%, and water for injection, q.s.
Doxapram is a respiratory stimulant.
Doxapram hydrochloride is a white to off-white, crystalline powder, sparingly soluble in water, alcohol and chloroform. It has the following chemical structure and name:
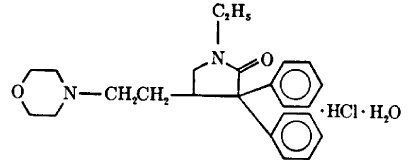
Molecular Formula: C24H30N2O2•HCl•H2O M. W. = 432.98
(±)-1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone monohydrochloride monohydrate.
Doxapram - Clinical Pharmacology
Pharmacodynamics
Doxapram hydrochloride produces respiratory stimulation mediated through the peripheral carotid chemoreceptors. As the dosage level is increased, the central respiratory centers in the medulla are stimulated with progressive stimulation of other parts of the brain and spinal cord.
The onset of respiratory stimulation following the recommended single intravenous injection of Doxapram hydrochloride usually occurs in 20 to 40 seconds with peak effect at 1 to 2 minutes. The duration of effect may vary from 5 to 12 minutes.
The respiratory stimulant action is manifested by an increase in tidal volume associated with a slight increase in respiratory rate.
A pressor response may result following Doxapram administration. Provided there is no impairment of cardiac function, the pressor effect is more marked in hypovolemic than in normovolemic states. The pressor response is due to the improved cardiac output rather than peripheral vasoconstriction...



