Dyural-80
Generic name: methylprednisolone acetate, lidocaine hydrochloride, bupivacaine hydrochloride, povidine iodine, isopropyl alcohol
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Methylprednisolone Acetate Injectable Suspension, USP
80 mg/mL (1 mL)
Rx only
Single-Dose Vial
Not For Intravenous Use
Dyural-80 Description
Methylprednisolone acetate injectable suspension, USP is an anti-inflammatory glucocorticoid for intramuscular, intra-articular, soft tissue or intralesional injection. It is available as single-dose vials in 80 mg/mL strength
Each mL of these preparations contains:
## | 80 mg/mL |
Methylprednisolone Acetate, USP | 80 mg |
Polyethylene glycol 3350 | 28 mg |
Myristyl-gamma-picolinium chloride | 0.189 mg |
Sodium chloride was added to adjust tonicity.
When necessary, pH was adjusted with sodium hydroxide and/or hydrochloric acid.
The pH of the finished product remains within the USP specified range (e.g., 3.0 to 7.0).
The chemical name for methylprednisolone acetate is pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-6-methyl-,(6α,11β)- and the molecular weight is 416.51. The structural formula is represented below:
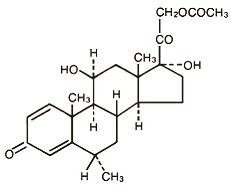
Methylprednisolone acetate injectable suspension, USP contains methylprednisolone acetate, USP which is the 6-methyl derivative of prednisolone. Methylprednisolone acetate, USP is a white or almost white crystalline powder which melts at about 213° with some decomposition. It is soluble in dioxane, sparingly soluble in acetone, alcohol, chloroform, and methanol, and slightly soluble in ether. It is practically insoluble in water.



