Epifoam
Generic name:pramoxine hydrochloride and hydrocortisone acetate
Dosage form: aerosol, foam
Drug class:Anorectal preparations
Medically reviewed by Drugs.com. Last updated on Sep 1, 2020.
On This Page
DESCRIPTION
Epifoam® (hydrocortisone acetate 1% and pramoxine hydrochloride 1%) is a topical aerosol foam containing: hydrocortisone acetate 1% and pramoxine hydrochloride 1% in a base containing: propylene glycol, cetyl alcohol, glyceryl monostearate and PEG 100 stearate blend, laureth-23, polyoxyl-40 stearate, methylparaben, propylparaben, trolamine, purified water and inert propellants: isobutane and propane.
Epifoam® contains a synthetic corticosteroid used as an anti-inflammatory/antipruritic agent and a local anesthetic.
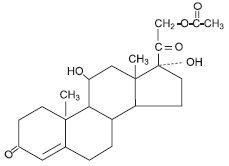
Hydrocortisone acetate
Molecular weight: 404.50. Solubility of hydrocortisone acetate in water: 1mg/100mL.
Chemical name: Pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β)-.
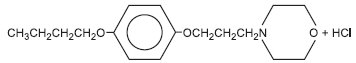
Pramoxine hydrochloride
Molecular weight: 329.86. Pramoxine hydrochloride is freely soluble in water.
Chemical name: morpholine, 4-[3-(4- butoxyphenoxy) propyl]-, hydrochloride.
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pramoxine Hydrochloride
A surface or local anesthetic which is not chemically related to the “caine” types of local anesthetics. Its unique chemical structure is likely to minimize the danger of cross-sensitivity reactions in patients allergic to other local anesthetics.