Esgic Tablets
Generic name:butalbital, acetaminophen and caffeine
Dosage form: tablet
Drug class:Analgesic combinations
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
The Esgic brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Warning
HEPATOTOXICITY
ACETAMINOPHEN HAS BEEN ASSOCIATED WITH CASES OF ACUTE LIVER FAILURE, AT TIMES RESULTING IN LIVER TRANSPLANT AND DEATH. MOST OF THE CASES OF LIVER INJURY ARE ASSOCIATED WITH THE USE OF ACETAMINOPHEN AT DOSES THAT EXCEED 4000 MILLIGRAMS PER DAY, AND OFTEN INVOLVE MORE THAN ONE ACETAMINOPHEN-CONTAINING PRODUCT.
Esgic Tablets Description
Butalbital, acetaminophen and caffeine are supplied in tablet form for oral administration.
Each tablet contains the following active ingredients:
Butalbital .....50 mg
Warning: May be habit-forming.
Acetaminophen .....325 mg
Caffeine .....40 mg
In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, crospovidone, microcrystalline cellulose, povidone, pregelatinized corn starch and stearic acid.
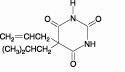
Butalbital (5-allyl-5-isobutylbarbituric acid), is a short to intermediate acting barbiturate. It has the following structural formula:
Acetaminophen (4'-hydroxyacetanilide), is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula: