Esidrix
Generic name:hydrochlorothiazide
Dosage form: Tablets
Drug class:Thiazide diuretics
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Esidrix®
hydrochlorothiazide USP
Tablets
Prescribing Information
On This Page
Esidrix Description
Esidrix, hydrochlorothiazide USP, is a diuretic and antihypertensive available as 25-mg and 50-mg tablets for oral administration. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4- benzothiadiazine-7-sulfonamide 1,1-dioxide, and its structural formula is
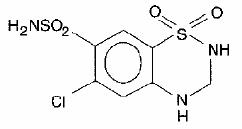
Hydrochlorothiazide USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water, freely soluble in sodium hydroxide solution, in n - butylamine and in dimethylformamide, sparingly soluble in methanol, and insoluble in ether, in chloroform, and in dilute mineral acids. Its molecular weight is 297.73.
Inactive Ingredients. Colloidal silicon dioxide, D&C Yellow No. 10 (50-mg tablets), FD&C Red No. 40 and FD&C Yellow No. 6 (25- mg tablets), lactose, starch, stearic acid, and sucrose.
Esidrix - Clinical Pharmacology
Thiazides affect the renal tubular mechanisms of electrolyte reabsorption. At maximal therapeutic dosage all thiazides are approximately equal in their diuretic potency. Thiazides increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium.
The mechanism of the antihypertensive effect of thiazides is unknown. Thiazides do not affect normal blood pressure.
Onset of action of thiazides occurs in 2 hours and the peak effect at about 4 hours. Its action persists for approximately 6 to 12 hours. Thiazides are eliminated rapidly by the kidney.
Indications and Usage for Esidrix
Hypertension
In the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe forms of hypertension.
Edema
As adjunctive therapy ...



