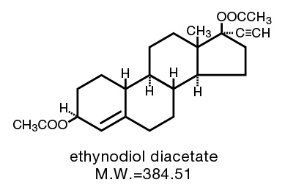
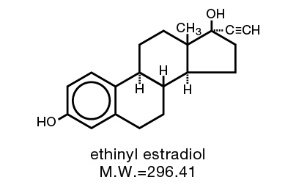
Ethynodiol diacetate and Ethinyl Estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
On This Page
Ethynodiol diacetate and Ethinyl Estradiol Description
Ethynodiol diacetate and Ethinyl Estradiol tablets USP, 1 mg/50 mcg. Each orange tablet contains 1 mg of ethynodiol diacetate and 50 mcg of ethinyl estradiol, and the inactive ingredients include lactose monohydrate, povidone K-25, microcrystalline cellulose, polacrilin potassium, magnesium stearate. In addition, the coloring agent is FD&C Yellow No. 6 Aluminum Lake. Each green tablet in the Ethynodiol diacetate and Ethinyl Estradiol tablets USP, 1 mg/50 mcg package is an inert tablet containing no active ingredients, and the inactive ingredients include lactose monohydrate, polacrilin potassium, magnesium stearate, yellow iron oxide and FD&C Blue No. 1 Aluminum Lake.
The chemical name for ethynodiol diacetate is 19-Nor-17α-pregn-4-en-20-yne-3β, 17-diol diacetate, and for ethinyl estradiol it is 19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol. The structural formulas are as follows:
Therapeutic class: Oral contraceptive.
Ethynodiol diacetate and Ethinyl Estradiol - Clinical Pharmacology
Combination oral contraceptives act primarily by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations in the genital tract, including changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation) may also contribute to contraceptive effectiveness.
Indications and Usage for Ethynodiol diacetate and Ethinyl Estradiol
Ethynodiol diacetate and Ethinyl Estradiol tablets USP, 1 mg/50 mcg are indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception. Oral contraceptive produ...