FaLessa
Generic name: folic acid
Dosage form: tablet
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
Physician Labeling
Rx only
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as chlamydia, genital herpes genital warts, gonorrhea, hepatitis B, and syphilis.
On This Page
FaLessa Description
21 orange active tablets each containing 0.1 mg of levonorgestrel d(-)-13β-hydroxygon-4-en-3-one, a totally synthetic progestogen, and 0.02 mg of ethinyl estradiol, 17α-ethinyl-17β-hydroxygon-4-en-3-one, a toally synthetic progestogen, and 0.02 mg of ethinyl estradiol, 17α-ethinyl-1,3,5(10)-estratriene-3, 17β-diol.
The chemical formula of levonorgestrel USP is 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17α)-, (-)-, and the chemical formula of ethinyl estradiol USP is 19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17α)-. The structural formulas are as follows:
The inactive ingredients present are FD&C Yellow #5 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, FD&C Red #40 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), iron oxide black, lactose monohydrate, magnesium stearate and pregelantinized corn starch.
Each inactive white tablet (7) contains the following inactive ingredients: titanium dioxide, polydextrose, hypromellose, triacetin, macrogol/polyethylene glycol 8000, lactose monohydrate, magnesium stearate and pregelantinized corn starch.
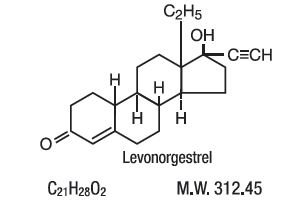
Levonorgestrel C21H28O2 MW: 312.4
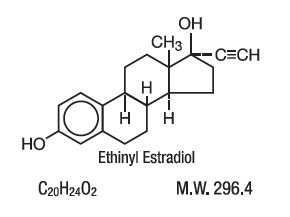
Ethinyl Estradiol C20H24O2 MW: 296.4
FaLessa - Clinical Pharmacology
Mode of action



