Flagyl Injection
Generic name:metronidazole
Dosage form: injection, solution
Drug classes:Amebicides, Miscellaneous antibiotics
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
On This Page
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Metronidazole Injection, USP RTU and other antibacterial drugs, Metronidazole Injection, USP RTU should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Warning
Metronidazole has been shown to be carcinogenic in mice and rats (see Precautions). Its use, therefore, should be reserved for the conditions described in the Indications and Usage section below.
Flagyl Injection Description
Metronidazole Injection, USP RTU, is a parenteral dosage form of the synthetic antibacterial agent 1-(ß-hydroxyethyl)-2-methyl-5-nitroimidazole.
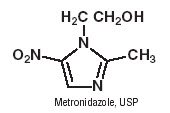
Metronidazole Injection, USP RTU, in 100 mL VIAFLEX Plus single dose plastic container, is a sterile, nonpyrogenic, iso-osmotic, buffered solution of 500 mg Metronidazole, USP, 790 mg Sodium Chloride, USP, 47.6 mg Dibasic Sodium Phosphate Dried, USP and 22.9 mg Citric Acid Anhydrous, USP. Metronidazole Injection, USP RTU has an osmolarity of 310 mOsmol/L (calc) and a pH of 5.5 (4.5 to 7.0). Each container contains 14 mEq of sodium.
The plastic container is fabricated from a specially formulated polyvinyl chloride plastic. Water can permeate from inside the container into the overwrap in amounts insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthala...



