Flextra 650
Generic name:acetaminophen and phenyltoloxamine citrate
Dosage form: tablet
Drug class:Analgesic combinations
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION
Each Tablet Contains:
Acetaminophen ..... 650 mg
Phenyltoloxamine
Citrate ..... 60 mg
Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
1.Acetaminophen
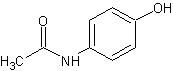
C8H2NO2 MW = 151.17
Phenyltoloxamine Citrate is a antihistamine having the chemical name N,N-dimethyl -2- (alpha-phenyl-o-tolyoxy) ethylamine dihydrogen citrate with the following chemical structure.
2. Phenyltoloxamine Citrate
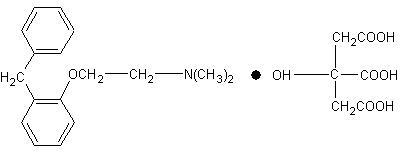
N, N-dimethyl-2 (alpha phenyl-ortho-tolxy)-ethyl-amine citrate
C17H21NO - C5H8O7 MW = 447.48
Inactive Ingredients
Each tablet contains microcrystaline cellulose, sodium starch glycolate, and magnesium stearate. Contains FD and C Red No. 40 Aluminum Lake and Orange Lake Blend
CLINICAL PHARMACOLOGY:
The analgesic action of Acetaminophen involves peripheral influences, but the specific mechanism is as yet undetermined. Antipyretic activity is mediated through hypothalamic heat regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of Acetaminophen have negligible effects on the cardiovascular or respiratory system; however, toxic doses may cause circulatory failure and rapid shallow breathing.
PHARMACOKINETICS:
The behavior of the individual components is described below.



