Flucaine
Generic name: fluorescein sodium and proparacaine hydrochloride
Dosage form: ophthalmic solution
Drug class:Ophthalmic diagnostic agents
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION:
Fluorescein Sodium and Proparacaine Hydrochloride Ophthalmic Solution, USP (Sterile) is a sterile ophthalmic solution combining the disclosing action of Fluorescein with the anesthetic action of Proparacaine Hydrochloride.
The active ingredient, Fluorescein Sodium, has the chemical name Spiro [isobenzofuran-1 (3H), 9'-[9H]xanthene]-3-one, 3' ,6' dihydroxy-,disodium salt. It is represented by the following structural formula:
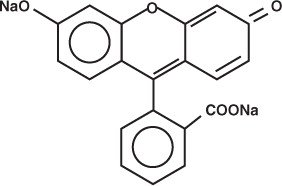
The active ingredient, Proparacaine Hydrochloride, has the chemical name Benzoic acid, 3-amino-4-propoxy-, 2-[diethylamino]ethyl ester monohydrochloride. It is represented by the following structural formula:
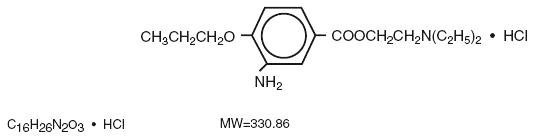
EACH mL CONTAINS: ACTIVES: Fluorescein Sodium, USP, 0.25% [2.5 mg]. Proparacaine Hydrochloride, USP, 0.5% [5mg]; INACTIVES: Povidone, Boric Acid, Waer for Injection, Sodium
PRESERVATIVE: Methylparaben 0.1%.
Flucaine - Clinical Pharmacology
This product is the combination of a disclosing agent with a rapidly acting anesthetic of short duration.
For procedures requiring a disclosing agent in combination with an anesthetic agent such as tonometry, gonioscopy, removal of corneal foreign bodies and other short corneal or conjunctival procedures.



