Gallium
Generic name: Gallium citrate Ga 67
Dosage form: Injection
Drug class:Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Rx Only.
Diagnostic – For Intravenous Use
Gallium Description
Gallium Citrate Ga 67 Injection is supplied in a 10 milliliter vial as an isotonic, sterile, non-pyrogenic solution. Each milliliter of the isotonic solution contains 74 megabecquerels (2 millicuries) of Gallium Ga 67 on the calibration date as a complex formed from 8.3 nanograms Gallium chloride Ga 67, 1.9 milligrams of sodium citrate dihydrate, 7.8 milligrams of sodium chloride and 0.9 percent benzyl alcohol (v/v) as a preservative. The pH is adjusted to between 5.5 to 8.0 with hydrochloric acid and/or sodium hydroxide solution.
Gallium Ga 67, with a half-life of 78.26 hours, is cyclotron produced by the proton irradiation of enriched zinc. At the time of calibration the drug contains no more than 0.02% Gallium Ga 66 and no more than 0.2% Zinc Zn 65. The concentration of each radionuclidic impurity changes with time. At expiration, the drug contains no more than 0.001% Gallium Ga 66 and no more than 1.0% Zinc Zn 65. No carrier has been added.
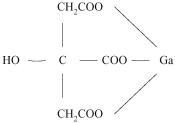
Gallium Citrate has the following chemical structure:
PHYSICAL CHARACTERISTICS
Gallium Ga 67 with a physical half-life of 78.26 hours1 decays by electron capture to stable Zinc Zn 67. Photons that are useful for imaging studies are listed in Table 1.
| Radiation | Mean % Per Disintegration | Energy |
| Gamma-2 | 2.9 |
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
|