Gantrisin
Generic name:acetyl sulfisoxazole
Dosage form: Pediatric Suspension
Drug class:Sulfonamides
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Gantrisin Description
Gantrisin (sulfisoxazole) is an antibacterial sulfonamide available as a pediatric suspension for oral administration. Each teaspoonful (5 mL) of the pediatric suspension contains the equivalent of approximately 0.5 gm sulfisoxazole in the form of acetyl sulfisoxazole in a vehicle containing 0.3% alcohol, carboxymethylcellulose (sodium), citric acid, methylcellulose, parabens (methyl and propyl), partial invert sugar, sodium citrate, sorbitan monolaurate, sucrose, flavors and water.
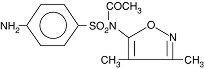
Acetyl sulfisoxazole, the tasteless form of sulfisoxazole, is N1-acetyl sulfisoxazole and must be distinguished from N4-acetyl sulfisoxazole, which is a metabolite of sulfisoxazole. Acetyl sulfisoxazole is a white or slightly yellow, crystalline powder that is slightly soluble in alcohol and practically insoluble in water. Acetyl sulfisoxazole has a molecular weight of 309.34 and the following structural formula:
Gantrisin - Clinical Pharmacology
Following oral administration, sulfisoxazole is rapidly and completely absorbed; the small intestine is the major site of absorption, but some of the drug is absorbed from the stomach. Sulfonamides are present in the blood as free, conjugated (acetylated and possibly other forms) and protein-bound forms. The amount present as "free" drug is considered to be the therapeutically active form. Approximately 85% of a dose of sulfisoxazole is bound to plasma proteins, primarily to albumin; 65% to 72% of the unbound portion is in the nonacetylated form.
Maximum plasma concentrations of intact sulfisoxazole following a single 2-gm oral dose of sulfisoxazole to healthy adult volunteers ranged from 127 to 211 mcg/mL (mean, 169 mcg/mL) and the time of peak plasma concentration ranged from 1 to 4 hours (mean, 2.5 hours). The elimination half-life of sulfisoxazole ranged from 4.6 to 7.8 hours after oral administration. The elimination of sulfisoxazole has been shown to be slower in elderly subjects (63 to 75 y...



