Gastrografin
Generic name:diatrizoate meglumine and diatrizoate sodium
Dosage form: oral, rectal liquid
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
Gastrografin Description
Gastrografin (Diatrizoate Meglumine and Diatrizoate Sodium Solution) is a palatable lemon-flavored water-soluble iodinated radiopaque contrast medium for oral or rectal administration only. Each mL contains 660 mg diatrizoate meglumine and 100 mg diatrizoate sodium; pH has been adjusted to 6.0 to 7.6 with sodium hydroxide. Each mL contains approximately 4.8 mg (0.21 mEq) sodium and 367 mg organically bound iodine. Inactive ingredients: edetate disodium, flavor, polysorbate 80, purified water, saccharin sodium, simethicone, and sodium citrate.
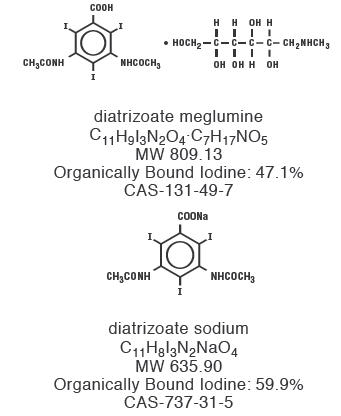
Diatrizoate meglumine is designated chemically as 1-deoxy-1-(methylamino)-D-glucitol 3,5-diacetamido-2,4,6-triiodo-benzoate (salt); diatrizoate sodium is monosodium 3, 5-diacetamido-2,4,6-triiodobenzoate. Structural formulas:
Gastrografin - Clinical Pharmacology
The most important characteristic of contrast media is the iodine content. The relatively high atomic weight of iodine contributes sufficient radiodensity for radiographic contrast with surrounding tissues.
Diagnostic enteral radiopaque agents have few known pharmacological effects. Diatrizoate meglumine and diatrizoate sodium exert a mild laxative effect attributable to their high osmolarity.
Diatrizoate meglumine and diatrizoate sodium are sparingly absorbed from the intact gastrointestinal tract, and therefore permit gastrointestinal opacification and delineation after oral or rectal administration. Oral administration is used for radiographic evaluation of the esophagus, stomach and proximal small intestine. Rectal administration is used for examination of the colon; however, visualization of the distal small bowel is generally unsatisfactory, since the hypertonicity of the medium causes intraluminal diffusion of water with subsequent dilution of the medium. Enough absorption from the gastrointestinal tract to permit incidental visualization of the urinary tract has been reported; this should also be considered when thyroid testi...



