Gastromark
Generic name: ferumoxsil
Dosage form: oral suspension
Drug class:Magnetic resonance imaging contrast media
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
Gastromark Description
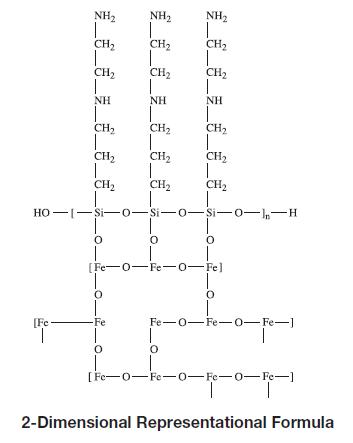
Gastromark™ (ferumoxsil, oral suspension) is an aqueous suspension of silicone-coated, superparamagnetic iron oxide, intended for oral administration as a magnetic resonance imaging contrast media. Gastromark is designated chemically as poly [N-(2-aminoethyl)-3-aminopropyl] siloxane coated non-stoichiometric magnetite (FeOx[C5H13N2SiO2]y), which has been manufactured to obtain a small uniform particle size of approximately 0.4 microns.
Gastromark is a turbid, slightly viscous, dark brown to orange-brown liquid formulation prepared for oral administration. The formulation contains water, sodium chloride, sorbitol, saccharin, carboxymethylcellulose, methylparaben, propylparaben, yellow dye #6, red dye #40, and flavoring. Each milliliter of Gastromark contains 175 micrograms of iron. Each milliliter also contains 1.4 milligrams of parabens as antimicrobial agents. The osmolality of the suspension is 250 mOsm; specific gravity is 1.01 grams per milliliter. The pH is 5.5 to 9.0, adjusted with sodium hydroxide.
The product is supplied in 360 mL bottles containing 300 mL of Gastromark.
Gastromark - Clinical Pharmacology
General
Gastromark is an oral aqueous suspension of a superparamagnetic magnetic resonance imaging (MRI) contrast agent. After oral administration of Gastromark, the agent fills the stomach and small intestine by 30 to 45 minutes after ingestion. The imaging agent passes distally to the large intestine by 4 to 7 hours after ingestion. Gastromark is primarily eliminated in the feces.
Pharmacokinetics
Absorption – Following the administration of 600 mL of Gastromark containing 10μCi of 59Fe (105 mg of iron) to 3 healthy, male volunteers and the same Gastromark dose containing 9.5μCi of 59Fe (105 mg of iron) to 3 male patients with inflammatory bowel diseases, the blood concentrat.



