Glofil-125
Generic name: iothalamate sodium I-125
Dosage form: injection, solution
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
Rx Only
Glofil-125 Description
General
Glofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per mL, and 0.9 percent benzyl alcohol as a preservative. The radioactive concentration of the material is 250-300 µCi/mL as of the calibration date. Sodium bicarbonate and hydrochloric acid are present for pH adjustment.
Physical Characteristics
Iodine-125 decays by electron capture with a physical half-life of 60.14 days.
Photons that are useful for detection are listed in Table 1.
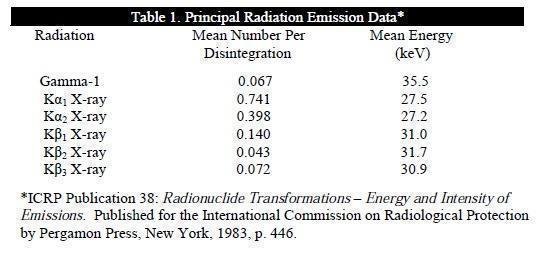
The specific gamma ray constant for I-125 is 1.43 R/mCi-hr at 1 cm. The first half value thickness of lead (Pb) for I-125 is 0.017 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide resulting from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.28 mm of Pb will decrease the external radiation exposure by a factor of 10,000.

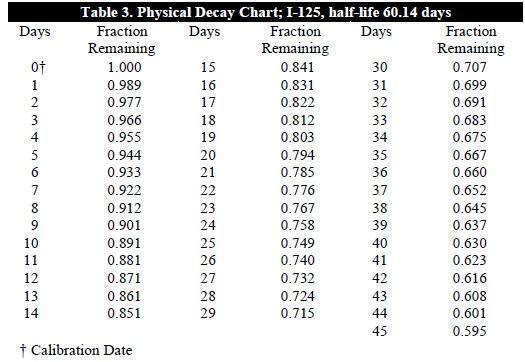
To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the date of calibration are shown in Table 3.
Glofil-125 - Clinical Pharmacology
The renal clearance of sodium iothalamate in man closely approximates that of inulin. The compound is cleared by glomerular filtration without tubular secretion or reabsorption. Following infusion administration of I-125 iothalamate, the effective half-life is about 0.07 days.
Indications and Usage for Glofil-125
Glofil-125 (Sodium Iothalamate I-125 Injection) is indicated for evaluation of glomerular filtration in the diagnosis or monitoring of patients with renal disease.
..


