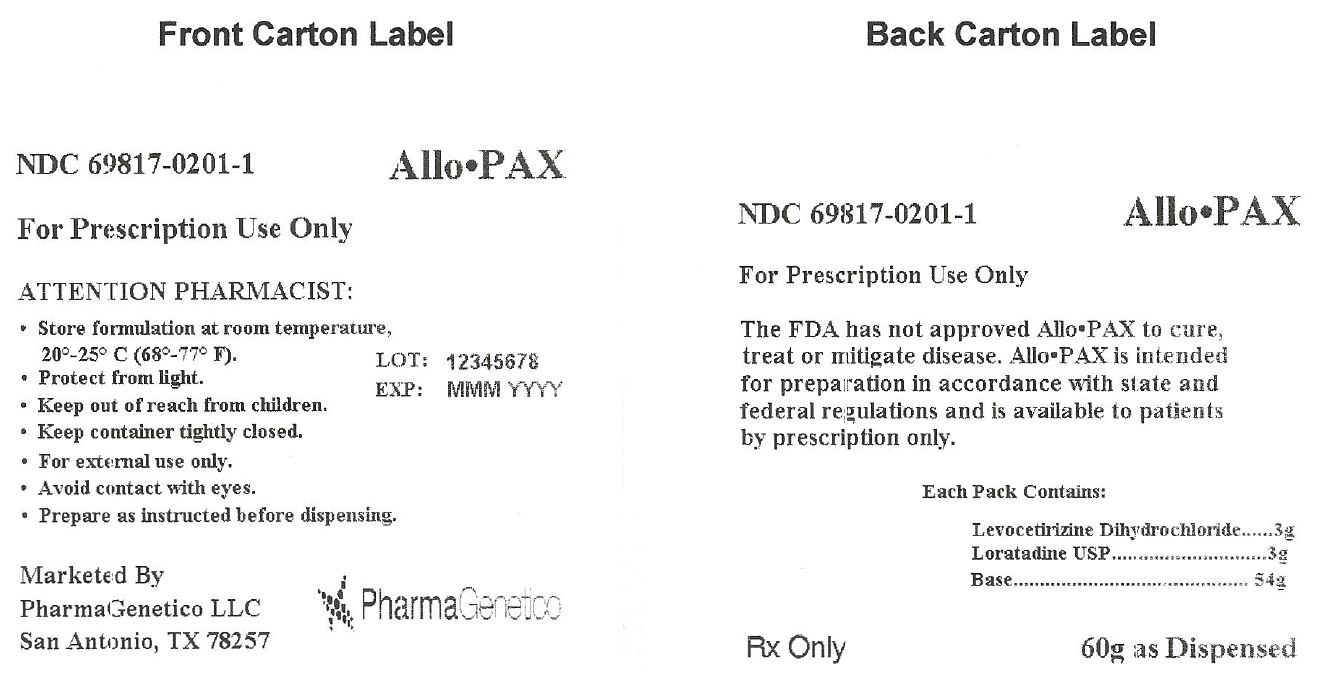
AlloPAX
Generic name: levocetirizine dihydrochloride, loratadine
Dosage form: topical application
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Each Allo•PAX provides 3 grams of Levocetirizine dihydrochloride, 3 grams Loratadine USP, and 54 grams of Base. The resulting mixture is intended for transdermal use.
For Prescription Use Only
Distributed by:
PharmaGenetico LLC
San Antonio, TX 78257

| AlloPAX levocetirizine dihydrochloride 5%, loratadine 5% kit | |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||



