Halfan
Generic name:halofantrine hydrochloride
Dosage form: tablets
Drug class:Miscellaneous antimalarials
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
Halfan HAS BEEN SHOWN TO PROLONG QTc INTERVAL AT THE RECOMMENDED THERAPEUTIC DOSE. THERE HAVE BEEN RARE REPORTS OF SERIOUS VENTRICULAR DYSRHYTHMIAS SOMETIMES ASSOCIATED WITH DEATH, WHICH MAY BE SUDDEN. Halfan IS THEREFORE NOT RECOMMENDED FOR USE IN COMBINATION WITH DRUGS OR CLINICAL CONDITIONS KNOWN TO PROLONG QTc INTERVAL, OR IN PATIENTS WHO HAVE PREVIOUSLY RECEIVED MEFLOQUINE, OR IN PATIENTS WITH KNOWN OR SUSPECTED VENTRICULAR DYSRHYTHMIAS, A-V CONDUCTION DISORDERS OR UNEXPLAINED SYNCOPAL ATTACKS. Halfan SHOULD BE PRESCRIBED ONLY BY PHYSICIANS WHO HAVE SPECIAL COMPETENCE IN THE DIAGNOSIS AND TREATMENT OF MALARIA, AND WHO ARE EXPERIENCED IN THE USE OF ANTIMALARIAL DRUGS. PHYSICIANS SHOULD THOROUGHLY FAMILIARIZE THEMSELVES WITH THE COMPLETE CONTENTS OF THIS LEAFLET BEFORE PRESCRIBING Halfan.
Halfan Description
Halfan (halofantrine hydrochloride) is an antimalarial drug available as tablets containing 250 mg of halofantrine hydrochloride (equivalent to 233 mg of the free base) for oral administration.
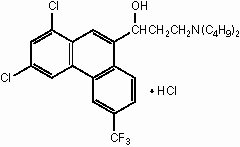
The chemical name of halofantrine hydrochloride is 1,3-dichloro-α-[2-(dibutylamino) ethyl]-6-(trifluoromethyl)-9-phenanthrene-methanol hydrochloride.
The drug, a white to off-white crystalline compound, is practically insoluble in water. Halofantrine hydrochloride has a calculated molecular weight of 536.89. The empirical formula is C26H30Cl2F3NO•HCl and the structural formula is
Inactive Ingredients
Inactive ingredients are magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, sodium starch glycolate, and talc.
Halfan - Clinical Pharmacology
The interindividual variability in the pharmacokinetic parameters of halofantrine is very wide and has led to great difficulty in ...



