Halonate
Generic name:halobetasol propionate
Dosage form: ointment
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
Description
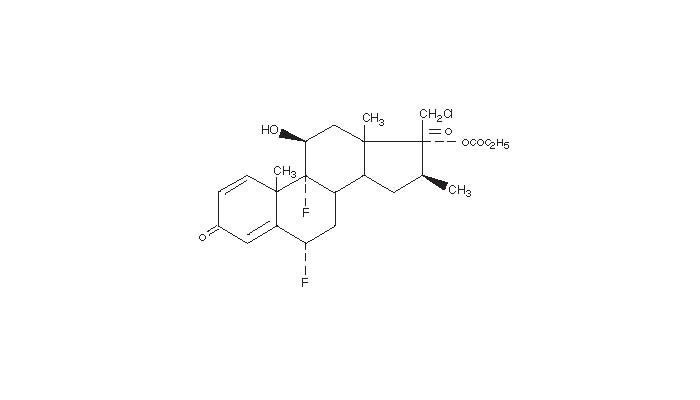
Halonate Halobetasol Propionate Ointment, 0.05% contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory and anti-pruritic agent. Chemically halobetasol propionate is 21-chloro-6α, 9-difluoro-11β,17-dihydroxy-16β-methylpregna-1, 4-diene-3-20-dione, 17-propionate, C25H31ClF2O5. It has the following structural formula:
base of aluminum stearate, beeswax, pentaerythritol cocoate, petrolatum, propylene glycol, sorbitan sesquioleate, and stearyl citrate.
Clinical Pharmacology
Like other topical corticosteroids, halobetasol propionate has anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of the anti-inflammatory activity of the topical corticosteroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.Human and animal studies indicate that less than 6% of the applied dose of halobetasol propionate enters the circulation within 96 hours following topical administration of the ointment. Studies performed with Halobetasol Propionate Ointment indicate that it is in the



