Hycomine
Generic name: hydrocodone bitartrate and phenylpropanolamine hydrochloride
Dosage form: Syrup
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
On This Page
Hycomine Description
Hycomine contains hydrocodone (dihydrocodeinone) bitartrate, a semisynthetic centrally-acting narcotic antitussive and phenylpropanolamine hydrochloride, a sympathomimetic amine decongestant for oral administration.
The pH of Hycomine and Hycomine Pediatric Syrup is 3.2-4.2. The hydrocodone component is (5α)-4,5-epoxy-3-methoxy-17-methylmorphinan-6-one [R-(R*,R*)]-2,3-dihydroxybutanedioate (1:1) hydrate (2:5), a fine white crystal or crystalline powder, which is derived from the opium alkaloid, thebaine, and has a molecular weight of 494.50. The phenylpropanolamine component is (±)-(R*,S*)-α-(1-aminoethyl) benzenemethanol hydrochloride and has a molecular weight of 187.67. These may be represented by the following structural formulas:
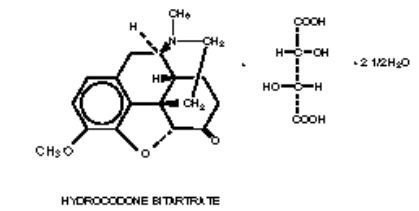
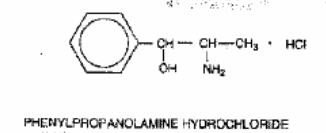
Hycomine
Pediatric Syrup
Each teaspoonful (5 mL) contains:
Hydrocodone bitartrate, USP 2.5 mg
WARNING: May be habit forming
Phenylpropanolamine hydrochloride, USP 12.5 mg
Hycomine Syrup
Each teaspoonful (5 mL) contains:
Hydrocodone bitartrate, USP 5 mg
WARNING: May be habit forming
Phenylpropanolamine hydrochloride, USP 25 mg
Also, Hycomine, both strengths, contain: artificial cherry flavor, glycerin, methylparaben, propylparaben, saccharin sodium, and sorbitol solution. Hycomine Pediatric Syrup contains: D&C Yellow 10 and FD&C Green 3. Hycomine Syrup: FD&C Red 40 and FD&C Yellow 6.
Hycomine - Clinical Pharmacology
Hydrocodone is a semisynthetic narcotic antitussive and analgesic with multiple actions qualitatively similar to those



