Hydro 35 Foam
Generic name:urea in a water and lipid based foam containing lactic acid
Dosage form: aerosol, foam
Drug class:Topical emollients
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
For Topical Dermatological Use Only
Rx Only - Caution: Federal Law restricts this product to sale by, or on the order of a licensed healthcare practitioner.
On This Page
Hydro 35 Foam Description
Hydro 35 Foam is a keratolytic agent delivered in a water & lipid based emollient foam containing lactic acid. This foam gently softens excess tissue to enhance removal from skin and nails, while rehydrating healthy tissue. Each gram contains 35% Urea as the active ingredient.
| CHEMICAL STRUCTURE | 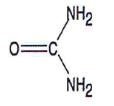 |
| Urea has the following chemical structure: |
Hydro 35 Foam - Clinical Pharmacology
Topically applied urea dissolves the intercellular matrix of the skin which results in softening of the hyperkeratotic tissue, and thus enhances shedding of scaly, dry skin. Urea topically applied to the nail plate has a similar effect on the intercellular matrix of the nail plate.
PHARMACOKINETICS
The mechanism of action of topical urea is not yet known.
INDICATIONS FOR USE
For enzyme debridernent and promotion of normal healing of surface lesions, particularly where healing is retarded by local infection. Necrotic tissue. fibrinous or purulent debris. Or eschar. Topically applied urea is useful for the treatment of hyperkeratotic conditions such as dermatitis. psoriasis, xerosis.ichthyosis, eczema.keratosis.keratoderma, and dry, rough skin, as well as corns and calluses and damaged, ingrown and devitalized nails.
Contraindications
Hydro 35 Foam should not be used by persons who ...



