Hydrocortisone and Acetic Acid
Dosage form: otic solution
Drug class:Otic steroids with anti-infectives
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
On This Page
Rx Only
Hydrocortisone and Acetic Acid Description
Hydrocortisone and Acetic Acid Otic Solution USP, is a solution containing hydrocortisone USP, (1%) and acetic acid USP, (2%), in a propylene glycol vehicle containing propylene glycol diacetate (3%), benzethonium chloride (0.02%), sodium acetate (0.015%), and citric acid (0.2%). The molecular formulas for acetic acid, USP and hydrocortisone, USP are C2H4O2 and C21H30O5, with molecular weights of 60.05 and 362.46, respectively. The structural formulas are:
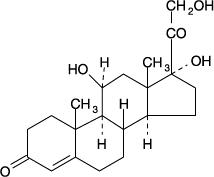
Chemically, hydrocortisone, USP is: Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-(11ß)-.
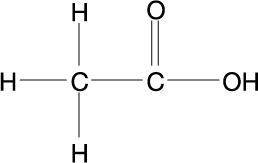
Acetic Acid, USP
This product is available as a nonaqueous otic solution buffered at pH 2 to 4 for use in the external ear canal.
Hydrocortisone and Acetic Acid - Clinical Pharmacology
Acetic acid is anti-bacterial and anti-fungal; hydrocortisone is anti-inflammatory, anti-allergic and anti-pruritic; propylene glycol is hydrophilic and provides a low surface tension; benzethonium chloride is a surface active agent that promotes contact of the solution with tissues.
Indications and Usage for Hydrocortisone and Acetic Acid
For the treatment of superficial infections of the external auditory canal caused by organisms susceptible to the action of the antimicrobial, complicated by inflammation.
Contraindications
Hypersensitivity to any of the ingredients, herpes simplex, vaccinia and varicella. Perforated tympanic membrane is considered a contraindication to the use of any medication in the external ear canal.
Warnings
Discontinue promptly if sensitization or irritation occurs.



