Hydrocortisone and Pramoxine Cream
Generic name: hydrocortisone acetate and pramoxine hydrochloride
Dosage form: cream
Drug class:Anorectal preparations
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Rx Only
For External Use Only. Not for Ophthalmic Use.
Hydrocortisone and Pramoxine Cream Description
Hydrocortisone Acetate 2.5% and Pramoxine HCl 1% Cream is a topical preparation containing hydrocortisone acetate 2.5% w/w and pramoxine hydrochloride 1% w/w in a hydrophilic cream base containing cetyl alcohol, stearyl alcohol, glycerin, glyceryl stearate SE, mineral oil, PEG-100 stearate, phenoxyethanol, purified water, white petrolatum, and xanthan gum. Topical corticosteroids are anti-inflammatory and anti-pruritic agents. The structural formula, the chemical name, molecular formula and molecular weight for the active ingredients are presented below
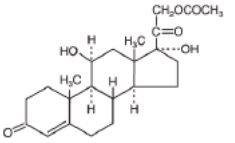
hydrocortisone acetate
Pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11,
17-dihydroxy-, (11-beta)-
C 23H 32O 6; mol. wt: 404.50
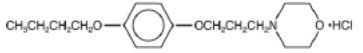
pramoxine hydrchloride
4-(3-(butoxyphenoxy)propyl)morpholine hydrochloride
C 17H 27NO 3.HCl; mol. wt: 329.87
Hydrocortisone and Pramoxine Cream - Clinical Pharmacology
Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency...



