Hydroquinone Cream
Dosage form: cream
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Mar 1, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
HYDROQUINONE USP, 4%
Skin Bleaching Cream
Rx Only
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
Hydroquinone Cream Description
Each gram of HYDROQUINONE USP, 4% SKIN BLEACHING CREAM contains 40 mg hydroquinone, in a cream base of Glyceryl Monostearate, Mineral Oil, PEG-25 Propylene Glycol Stearate, Polyoxl-40 Stearate, Propylene Glycol, Propylparaben, Purified water, sodium metabisulfite, Squalane and Stearic Acid.
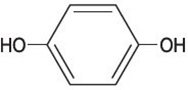
Chemically, hydroquinone is C 6H 6O 2 and has a molecular weight of 110.11. The chemical name is 1,4 dihydroxybenzene, and the structural formula of hydroquinone is:
Hydroquinone Cream - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (dopa) (Denton, C. et al., 1952) 1 and suppression of other melanocyte metabolic processes (Jimbow, K. et al., 1974) 2. Exposure to sunlight or ultraviolet light will cause repigmentation of bleached areas (Parrish, J.A. et al., 1978) 3.
Indications and Usage for Hydroquinone Cream
HYDROQUINONE USP, 4% SKIN BLEACHING CREAM is indicated for the gradual bleaching of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.



