Hydroquinone Time Release Cream
Dosage form: cream
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Skin Lightening Moisturizing Cream
Rx Only
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
Hydroquinone Time Release Cream Description
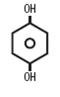
Each gram of Hydroquinone USP, 4% Time Release Cream contains 40 mg hydroquinone USP and retinyl palmitate (vitamin A) incorporated into microspheres composed of acrylates/C10-30 alkyl acrylate crosspolymer. This polymeric system provides gradual release of active ingredient into the skin. Other ingredients include ascorbic acid (vitamin C), ascorbyl palmitate, ascorbyl tetraisopalmitate, benzyl alcohol, bisabolol, butylated hydroxytoluene, cetyl alcohol, cyclopentasiloxane, edetate disodium, ethylhexyl palmitate, glycerin, glyceryl monostearate, light mineral oil, phenoxyethanol, poloxamer 188, polyoxyl 40 stearate, propyl gallate, purified water, Simmondsia chinensis (Jojoba) seed oil, sodium metabisulfite, sorbitan tristearate, tocopheryl acetate (vitamin E), tricontanyl PVP, and trolamine. Chemically, hydroquinone is C6H6O2 and has a molecular weight of 110.11. The chemical name is 1,4 dihydroxybenzene, and the structural formula of hydroquinone is:
Hydroquinone Time Release Cream - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydr...