Altacaine Ophthalmic Solution
Generic name: tetracaine hydrochloride
Dosage form: ophthalmic solution
Drug class:Ophthalmic anesthetics
Medically reviewed by Drugs.com. Last updated on Dec 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
TetracaineHydrochloride Ophthalmic
Solution, USP 0.5% Sterile
Rx Only
On This Page
DESCRIPTION:
Tetracaine Hydrochloride 0.5% is a sterile topical ophthalmic solution useful in producing surface anesthesia of the eye. The active ingredient is represented by the structural formula:
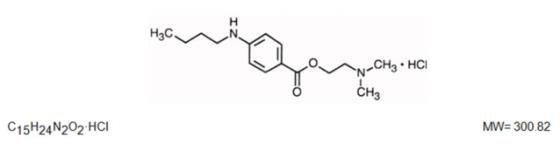
Established name:
Tetracaine Hydrochloride
Chemical name:
Benzoic acid, 4-(butylamino)-,2-(dimethylamino)ethyl ester, monohydrochloride
CONTAINS:
Active: Tetracaine Hydrochloride 0.5%; Preservative: Chlorobutanol; Inactive: Boric Acid, Edetate Disodium, Potassium Chloride, Water for Injection, USP. Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH.
CLINICAL PHARMACOLOGY:
Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% acts by decreasing the permeability of the neuronal membrane, thereby decreasing the flux of sodium, potassium and other ions associated with propagation of the nerve impulse. The onset of anesthesia usually begins within 30 seconds and lasts a relatively short period of time.
INDICATIONS AND USAGE:
For procedures in which a rapid and short acting topical ophthalmic anesthetic is indicated such as in tonometry, gonioscopy, removal of corneal foreign bodies, conjunctival scraping for diagnostic purposes, suture removal from the cornea or conjunctiva, other short corneal and conjunctival procedures.
CONTRAINDICATIONS:
Should not be used by the patient without physician supervision, or in those persons showing hypersensitivity to any component of this preparation.
...


